-
Products
-
Lab Instruments
Other Instruments
-
Lab Meters and Probes
Calibration Standards Radiometer ProbesOther Reagents
- Chemistries, Reagents, and Standards
-
Online Analysers
Ammonium Analysers Ammonia Monochloramine Chlorine Analysers
- CL17sc
- CL10sc Amperometric
- 9184 sc Amperometric
- Ultra Low Range CL17sc Colorimetric Chlorine Analyser
EZ Series Analysers- Iron
- Aluminium
- Manganese
- Phosphate
- Chloride
- Cyanide
- Fluoride
- Sulphate
- Sulphide
- Arsenic
- Chromium
- Copper
- Nickel
- Zinc
- Ammonium
- Total Nitrogen
- Total Phosphorus
- Phenol
- Volatile Fatty Acids
- Alkalinity
- ATP
- Hardness
- Toxicity
- Sample Preconditioning
- Boron
- Colour
- Nitrate
- Nitrite
- Silica
- Hydrogen Peroxide
- EZ Series Reagents
- EZ Series Accessories
- EZ sc Series Inorganics
- EZ sc Series Metals
- EZ sc Series Nutrients
-
Online Sensors and Controllers
Digital Controllers (Transmitters) Controllers (Analogue)
- SC4500
- Orbisphere 366x Ex
- Orbisphere 410/510 Carbon Dioxide
- Orbisphere 410/510 Oxygen
- Orbisphere 410/510 Ozone
- Orbisphere 51x Hydrogen
Single, Dual, Multi-parameter Online Panels pH & ORP Sensors- 1200-S ORP
- 1200-S pH
- 12mm pH/ORP
- 8362 sc High Purity
- Combination pH/ORP
- Differential pH
- Digital Differential ORP
- Digital Differential pH
- LCP ORP
- LCP pH
Conductivity Sensors- 3400 Analogue Contacting
- 3400 Digital Contacting
- 3700 Analogue Inductive
- 3700 Digital Inductive
- 3798 sc Electrodeless
- 9523 Cation Conductivity
- 9525 DCCP System
-
Automated Lab Systems
Robot Systems
- Multiparameter Online Panels
- Claros Water Intelligence System
- Samplers
- Test Kits & Strips
-
Lab Equipment and Supply
Apparatus
- Brushes
- Clamps, Rings & Stands
- Crucibles
- Crucibles & Casseroles
- Dispensers & Droppers
- Grab Samplers
- Oil and Grease
- Other Apparatus
- Pipet Aids
- Pipettes
- Racks
- Stir Bars
- Tubing
- Weighing Accessories
General Lab Consumables Glassware/PlasticwareInstruments -
Microbiology
Accessories and Chemicals Dehydrated MediaLabware
- Electrochemistry
-
Lab Instruments
- Parameters
-
Software Solutions
-
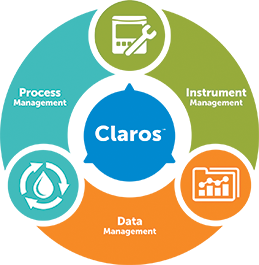
Claros Water Intelligence System
Product Pillars Process Management
- Solutions For:
- BOD/COD Removal
- Nitrification/Denitrification
- Phosphorous Removal
- Sludge Management
Data Management- Solutions For:
- Collection
- Visualization & Analytics
- Reporting
- Data Accuracy
Instrument Management- Solutions For:
- Maintenance
- Troubleshooting
- Remote Access
- Lab and Process Comparison
Industry Challenges Regulatory Compliance Cost Savings Remote Operations Process Optimisation Equipment Maintenance
-
Claros Water Intelligence System
- Industries
- Service
- News & Events
Ireland
Choose your country or region:
Europe
Americas
Asia - Australasia
- Australia
- Mainland China
- India
- Indonesia
- Japan
- Malaysia
- New Zealand
- Philippines
- Singapore
- South Korea
- Thailand (Thai)
- Taiwan
- Vietnam
Middle East - Africa
Phosphorus
What is Phosphorus?
Phosphorus (P) is a chemical element with an atomic number of 15 and an atomic mass of 31. Due to its high reactivity, phosphorus does not naturally exist as a free element. Phosphorus generally occurs as phosphate in minerals. Phosphate is typically found in the Earth’s crust at a concentration of about 1 gram per kilogram.
The two types of elemental phosphorus are white phosphorus and red phosphorus. When exposed to oxygen, white phosphorus emits a faint glow caused by oxidation (also known as chemiluminescence).
Phosphorus belongs to the pnictogen group of elements which consists of nitrogen, phosphorus, arsenic, antimony, bismuth and ununpentium (recently assigned the name moscovium). These elements are grouped because of their similar atomic structure, which lends them the ability to form stable compounds due to their tendency to form double and triple covalent bonds. Except for nitrogen (which remains a gas), the other pnictogens are solids at room temperature.
Complex organisms such as plants and animals need phosphorus because phosphates are a component of DNA, RNA, ATP and phospholipids. Phosphorus is mined for use in detergents, pesticides, nerve agents and predominantly fertilizers.
Phosphates/Orthophosphate
In orthophosphate (one of the most common phosphates), one phosphorus atom is bonded to four oxygen atoms. Orthophosphate is sometimes called “reactive phosphorus” because it bonds easily with other electron-deficient elements and compounds since the three “extra” electrons on the oxygen atoms strongly want to bond with protons alike.
Condensed Phosphates
Condensed phosphates are multiple orthophosphate molecules “condensed” together and sharing a covalent bond between adjoining phosphorus (P) and oxygen (O) atoms. This group includes metaphosphate, pyrophosphate and polyphosphate — which are often used for corrosion control in drinking water distribution systems.
Total Phosphorus/Organic Phosphorus
Total phosphorus is a sum of all the phosphorus present: orthophosphate/phosphates, condensed phosphates and organic phosphorus. Organic phosphorus is usually present in the form of phosphates contained inside or bonded to an organic compound.
Why Measure Phosphorus?
While phosphates don’t contribute to taste and odour issues, and, therefore, may not be a target in drinking water, high phosphate levels in wastewater discharge can have a significant impact on the surrounding ecosystem. High levels of phosphates in source water can accelerate types of algae and plant growth; this can lead to eutrophication and algae blooms. When this occurs, fish and aquatic life are robbed of oxygen, resulting in large fish kills and destroyed habitats.
Measuring Phosphate in Wastewater is Critical for a Healthy Ecosystem
Measuring phosphate in wastewater effluents is critical to maintaining a healthy ecosystem and protecting wildlife. Many areas have strict discharge limits for phosphates to protect the ecosystem that receives this water. Besides protecting the ecosystem, failing to monitor and control your phosphate levels can also lead to violations and fines.
Drinking water plants that use phosphates for corrosion control may need to monitor phosphates in finished water, distribution system and other stages of the treatment process. Hach ® solutions are designed to keep you in compliance and give your operators the knowledge they need to make treatment decisions.
At Hach, find the testing equipment, resources, training and software you need to successfully monitor and manage phosphorus levels in your specific process application.
Featured Products to Measure Phosphorus
The NP6000sc grab sample feature ensures compliance with regulatory standards while maintaining precise process control. With trusted, accurate readings, you can keep operations running smoothly. Plus, the NP6000sc has a lightweight, integrated FX6 filtration system option for reliable functionality and reduced touchpoints.
EZ Series Phosphate & Total Phosphorus Analyzers
The EZ Series Online Analyzers offer multiple options to monitor total phosphate in water.
The 5500 sc Phosphate Analyzer requires only 2 liters of reagent for the analyzer to perform unattended for up to 90 days; twice as long as the predecessor Series 5000 instruments.
Hach is an innovator in spectrophotometry and offers many of the leading spectrophotometric instruments in the water analysis market.
Whether you are looking for a single parameter testing device such as the DR300, or a more sophisticated hassle-free instrument like the DR900 that can measure many parameters with up to 90 methods, we have a solution.
The Hach digital thermostats is designed to save time and ensure accuracy during testing.
Which Processes Require Phosphorus Monitoring?
Drinking Water
Source water may contain elevated concentrations of phosphates due to agricultural runoff, which may cause eutrophication of surface water with algae growth and release of cyanotoxins. Condensed phosphates are often used for corrosion control in drinking water distribution systems, therefore drinking water treatment processes may require monitoring for phosphate in both raw and distributed finished water.

Wastewater Treatment
Phosphorus is an essential nutrient for plants and specifically for algae growth. The discharge of phosphorus, along with other essential nutrients in the wastewater effluent, stimulates algae growth in the streams receiving the wastewater discharge. Algae causes taste and odour problems, is aesthetically unpleasing and most importantly, creates enormous oxygen demand when algal bloom dies off. The depletion of oxygen caused by the dying algal blooms is toxic to fish and causes other significant disruptions to the aquatic environment. For these reasons, regulatory agencies often strictly regulate the amount of phosphorus allowed in wastewater discharges.
The most common methods for removing phosphorus from wastewater are biological removal and chemical precipitation. These processes are typically referred to as tertiary treatment and require phosphorus monitoring.

How is Phosphorus Monitored?

Colormetric Methods (Orthophosphate)
Orthophosphate (reactive phosphorus) is combined with molybdate in an acidic medium to produce either a blue or yellow colour. Be aware that no analytical test is perfect and some condensed phosphates may be measured with these tests too. Due to the acidic chemistry, some suspended orthophosphate may be detected if the sample was not first filtered to 0.45 micron.
To measure all phosphorus including the particulate phosphates, it is necessary to use a total phosphorus test, which incorporates rigorous digestion to convert most of the particulate phosphate to dissolved format.
Benchtop/Portable:
LCK349 Phosphate (Ortho/Total) cuvette test 0.05-1.5 mg/L PO₄-P
LCK348 Phosphate (Ortho/Total) cuvette test 0.5-5.0 mg/L PO₄-P
LCK350 Phosphate (ortho/total) cuvette test 2.0 -20.0 mg/L PO₄-P
LCK049 Orthophosphate cuvette test 1.6-30 mg/L PO₄-P
Phosphate Reagent Powder Pillows 0.02-2.50mg/L PO₄
Phosphate, ortho, TNT, 0.06 - 5.00 mg/L PO₄
Ortho Phosphate, TNT, 1.0 - 100 mg/L PO₄
Low Range Orthophosphate Chemkey Reagents (PPA)
High Range Orthophosphate Chemkey Reagents (PPA)
Online:

Acid Hydrolyzable Phosphate/Condensed Phosphate
To measure condensed phosphates, it is first necessary to transform them into orthophosphate using sulfuric acid and heat, digesting the sample at 150°C for 30 minutes. This is called “acid hydrolyzable phosphate” since the condensed phosphates are hydrolyzed into orthophosphate. After the digestion, either the ascorbic acid or molybdovanadate methods are used to measure the orthophosphate. Some organic phosphate will be hydrolyzed into orthophosphate as well, so the results are not “pure” condensed phosphate.
Of course, just performing the digestion and colorimetric test will tell you the concentration of both the original orthophosphate and condensed phosphates. If you want just the condensed phosphate concentration, then simply run the orthophosphate test on the same sample without a digestion and subtract those results from the first concentration value.
Total Phosphorus/Organic Phosphate
Organic phosphates are stubborn compounds that do not like to break down easily. To test for them, it is necessary to not only digest the sample first with sulfuric acid and heat, but also add a strong oxidant such as potassium persulfate to break the orthophosphates free from the organic bonds. After digestion, the same ascorbic acid or molybdovanadate methods can be used to measure the phosphate concentration. The described test will convert all the different forms of phosphate into orthophosphate, which means the results are total phosphorus.
If you want to know only the organically bound phosphate concentration, it is necessary to perform the acid hydrolyzable test and subtract those results from the total phosphorus concentration (total phosphorus concentrations will always be larger than the orthophosphate concentration).
Benchtop:
LCK349 Phosphate (Ortho/Total) cuvette test 0.05-1.5 mg/L PO₄-P
LCK348 Phosphate (Ortho/Total) cuvette test 0.5-5.0 mg/L PO₄-P
LCK350 Phosphate (ortho/total) cuvette test 2.0 -20.0 mg/L PO₄-P
Phosphorus, total, TNT, 0.06 - 3.5 mg/L PO₄
Phosphorus, total, TNT, 1.0 - 100 mg/L PO₄
Online:
Frequently Asked Questions
Can the Phosphax sc Analyzer be used in seawater applications?
No, the Phosphax sc cannot be used for seawater applications since this method works only up to 1000 mg/L chloride. The chloride content of seawater is typically above 19000 mg/L.
What is the difference between blue method and yellow methods for phosphates?
Reactive phosphorous can be measured colorimetrically using several different chemistries. First, the phosphorous reacts with molybdate in an acidic solution to form a phosphomolybdate complex. For low range measurements, the phosphomolybdate complex is reduced with either an amino acid or ascorbic acid, creating a characteristic molybdenum blue species. High range measurements can be made by reacting the phosphomolybdate complex with vanadium reagent to create a yellow-coloured product. Colour intensity of both the blue and yellow complexes are proportional to the concentration of phosphorous in the sample.
CUSTOMER SUPPORT
- Service
- Safety Data sheets (SDS)
- Certificates of Analysis
- Form for Ringtests
- COD/REACH
- CLAROS
- Certificate ISO 9001:2015
- Certificate ISO 14001:2015
- Certificate ISO 45001:2018
- Water Analysis Solutions – for Lab & Field
- Producer Register Limited (PRL)
Number: IE 0309 2 W B